Product Category
The Manufacturing Process
Most soft drinks are made at local bottling and canning companies. Brand name franchise companies grant licenses to bottlers to mix the soft drinks in strict accordance with their secret formulas and their required manufacturing procedures.
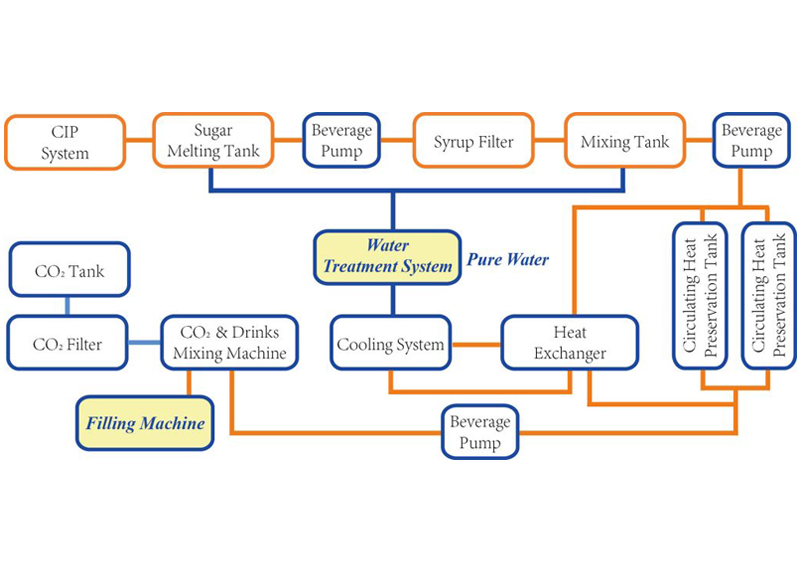
Request A QuoteThe quality of water is crucial to the success of a soft drink. Impurities, such as suspended particles, organic matter, and bacteria, may degrade taste and color. They are generally removed through the traditional process of a series of coagulation, filtration, and chlorination. Coagulation involves mixing a gelatinous precipitate, or floc (ferric sulfate or aluminum sulfate), into the water. The floc absorbs suspended particles, making them larger and more easily trapped by filters. During the clarification process, alkalinity must be adjusted with an addition of lime to reach the desired pH level.
The clarified water is poured through a sand filter to remove fine particles of floc. The water passes through a layer of sand and coarser beds of gravel to capture the particles.
Sterilization is necessary to destroy bacteria and organic compounds that might spoil the water's taste or color. The water is pumped into a storage tank and is dosed with a small amount of free chlorine. The chlorinated water remains in the storage tank for about two hours until the reaction is complete.
An activated carbon filter dechlorinates the water and removes residual organic matter, much like the sand filter. A vacuum pump de-aerates the water before it passes into a dosing station.
The dissolved sugar and flavor concentrates are pumped into the dosing station in a predetermined sequence according to their compatibility. The ingredients are conveyed into batch tanks where they are carefully mixed; too much agitation can cause unwanted aeration. The syrup may be sterilized while in the tanks, using ultraviolet radiation or flash pasteurization, which involves quickly heating and cooling the mixture.
The water and syrup are carefully combined by sophisticated machines, called proportioners, which regulate the flow rates and ratios of the liquids. The vessels are pressurized with carbon dioxide to prevent aeration of the mixture.
Carbonation is generally added to the finished product, though it may be mixed into the water at an earlier stage. The temperature of the liquid must be carefully controlled since carbon dioxide solubility increases as the liquid temperature decreases. Many carbonators are equipped with their own cooling systems. The amount of carbon dioxide pressure used depends on the type of soft drink. For instance, fruit drinks require far less carbonation than mixer drinks, such as tonics, which are meant to be diluted with other liquids. The beverage is slightly over-pressured with carbon dioxide to facilitate the movement into storage tanks and ultimately to the filler machine.
The finished product is transferred into bottles or cans at extremely high flow rates. The containers are immediately sealed with pressure-resistant closures, either tinplate or steel crowns with corrugated edges, twist off, or pull tabs.
Because soft drinks are generally cooled during the manufacturing process, they must be brought to room temperature before labeling to prevent condensation from ruining the labels. This is usually achieved by spraying the containers with warm water and drying them. Labels are then affixed to bottles to provide information about the brand, ingredients, shelf life, and safe use of the product. Most labels are made of paper though some are made of a plastic film. Cans are generally pre-printed with product information before the filling stage.
Finally, containers are packed into cartons or trays which are then shipped in larger pallets or crates to distributors.